During the 31st annual Transcatheter Cardiovascular Therapeutics scientific symposium, Santa Clara, CA-based Ancora presented interim study results from systolic heart failure patients treated with AccuCinch, a percutaneous therapy designed to improve left ventricular function.
A stronger and more experienced Ancora Heart entered into the 31st Transcatheter
Cardiovascular Therapeutics (TCT), the annual scientific symposium of the Cardiovascular Research Foundation this past week. The private company – which is poised to take on medtech giants Abbott Laboratories and Edwards Lifesciences in the functional mitral regurgitation (FMR) space, presented even more data than it did last year at TCT.
“There’s a big presence for us this year [at TCT] with three different presentations,” Jeff Closs, president and CEO of Ancora Heart, told MD+DI. “It is different in a very positive way this year vs. last year. I think it has to do with a couple of things. We’re further in our enrollment. We have more centers and more physicians who have now implanted the device and seen the patient results.”
The Santa Clara, CA-based company touted data from the CorCinch FMR study, a U.S. early feasibility study evaluating the safety of the investigational AccuCinch Ventricular Repair System designed for the treatment of systolic heart failure.
Enrollment in the CorCinch FMR study recently concluded and 35 patients were treated with the AccuCinch at 15 heart centers. The primary safety endpoint of the study is device-related or procedure-related major adverse events through 30 days. Secondary exploratory endpoints include technical success, device and procedural success, as well as other observational endpoints measuring heart function, heart failure symptoms, and changes in quality of life.
Data from the interim analysis indicate a favorable safety profile with 97% freedom from devicerelated major adverse events at 30 days. Preliminary efficacy data from the first nine patients treated with the latest implantation technique and with adjudicated core lab data available through six months demonstrated a reduction in left ventricular volume by an average of 23%.
Ejection fraction, a measure of blood flowing out of the left ventricle, improved on average from 31% to 39% over the same period. Additionally, Kansas City Cardiomyopathy Questionnaire scores increased by an average of 30%, suggesting reducing the left ventricular volume resulted in improved quality of life and reduced heart failure symptoms for this group. Further, mitral regurgitation grades and regurgitant volumes were both substantially reduced across this cohort.
“What was shown in the [FMR] study was a significant reduction in systolic volume,” Closs said. If you compare that to the COAPT data, which is from [Abbott’s] MitraClip, they report even when you fix the MR, they still report an increasing volume over time in the ventricle and a decreasing ejection fraction. The highlight of the data is what we’re showing on the ventricular side for FMR is quite different from any of the traditional valve-based approaches have shown.”
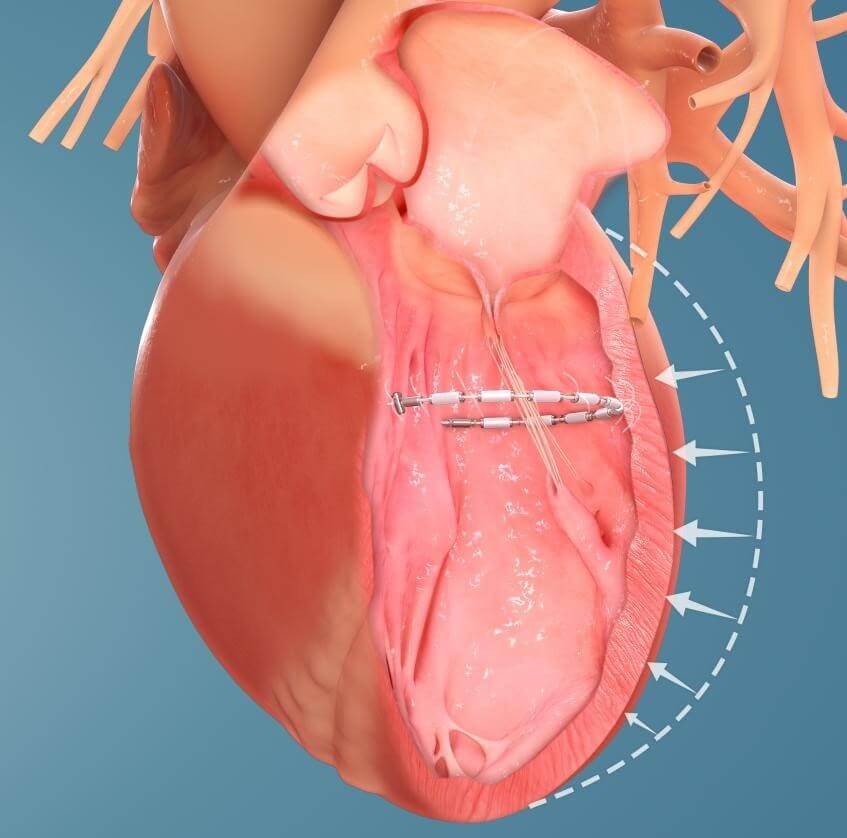
The transcatheter AccuCinch therapy is designed to complement and enhance the existing care cardiologists provide to further manage symptoms and slow, or stop, the progression of heart failure. For some patients, AccuCinch may have the potential to reverse the enlargement of the left ventricle. For patients where heart failure has progressed beyond the ability for medications and pacemakers to manage symptoms, non-surgical percutaneous device therapy with AccuCinch may provide an effective treatment option.
The AccuCinch system is designed to directly repair the left ventricle of the heart, thereby addressing the fundamental issue in the progression of systolic heart failure.
Ancora is currently vying for approval for AccuCinch. In May of last year, the company received approval from FDA to expand the enrollment for an early feasibility study of the AccuCinch.
“On the regulatory side our focus is on the U.S. IDE pivotal submission,” Closs said. “So, we’re finalizing the trial design as we speak, and we expect to file for the IDE sometime in the first quarter of next year and we would hope to begin enrollment in Q3 or Q4 of next year.”
Source:
https://www.mddionline.com/tales-tct-2019-ancora%E2%80%99s-heart-felt-data
